
Bioprocessing Single-Use Technologies - A Primer
According to BCC Research, the global single-use technology market will reach over $4.3 billion by 2021. The most significant increase will be in the highly adaptable...

Characteristics of Successful Medical Device Product Launches
The creation of a new medical device involves many steps from initial concept to finished product. Every year billions of dollars are invested in research and...

Is Aliphatic TPU Foam All it's Cracked Up To Be?
Polyurethane raw materials themselves have long seen use as dressings in the medical industry, but aliphatic thermoplastic polyurethane (aliphatic TPU) foams are gaining...
Advances in Medical Fiber-Optic Technology May Improve Quality of Care
Advances in medical fiber optics have proven useful in several areas of medical practice including urology, ophthalmology, and cardiology, to name just a few...
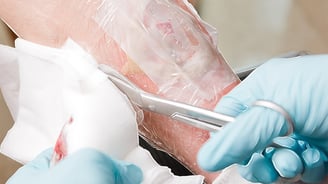
Moisture Management in Wound Care: Highlighting MVTR in Films
A wound can be described or defined in many ways: by its source, anatomical location and appearance, whether acute or chronic, by presenting symptoms, and by the method...

Anatomy of Films Used in Bioprocess Bags
In the early days of disposable medical devices, the biopharmaceutical industry manufactured single-use films made with "off-the-shelf" materials borrowed from other...

The Top Reasons Why Medical Devices Fail
Medical device failures are typically the result of deficiencies in safety check procedures or a lack of attention to potential risks in the design process. Often...

The Evolution of US Medical Device Regulation
Medical devices are regulated according to the same legislation as food and pharmaceuticals, under the general organization of the Food and Drug Administration (FDA)....

What's New With Nonwovens in the Medical Industry?
The utilization of nonwoven fabrics in the medical field has outpaced woven materials in recent years. Even when traced back to their rapid adoption during WWII,...

Applications of Flexible Materials in Healthcare
Flexible materials combined with emerging technologies are at the forefront of healthcare innovations. Guiding recent advances are cost concerns, mass-production, and...
%20-%20Tint%20100.png?width=106&name=Logo%20-%20Standard%20-%20Blue%20(270%20x%20149)%20-%20Tint%20100.png)