
7 Steps to Effectively Dual Source a Supply Chain for Medical Devices
Dual sourcing is considered a hassle by some traditional medical device manufacturers. After all, the procurement process is faster and more straightforward when you...

Development of Paper-Based Microfluidics for Point-of-Care Testing
Developments in the in vitro diagnostics (IVD) industry have been driven by global trends such as the prevalence of chronic diseases, an aging population, the increase...
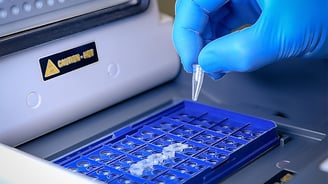
Application of Molecularly Imprinted Polymers in POC Diagnostics
Standard disposable point-of-care diagnostic (POCD) devices are making a positive impact on patient-based healthcare settings worldwide. These devices are convenient and...

6 Filtration Challenges in Downstream Biopharmaceutical Production
Membranes and membrane processes are efficient filtration tools in the manufacturing of biopharmaceutical products. These tools continue to evolve in response to new...
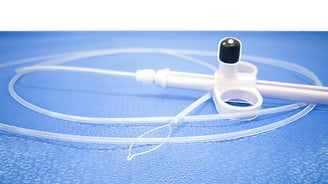
Single-Use Endoscopes: A Trend Towards Better Patient Safety
Design continues to save lives. The disposability designed into single-use medical devices such as surgical masks, syringes, and suction catheters has reduced the risk...
5 Things to Keep in Mind When Trying to Reverse Engineer Adhesive
The process of reverse engineering is demanding work: an analyst initially works with limited information about a product and uses that information to analyze it to...

Membrane Device Development for In Vitro Diagnostics
The surging trends of an aging global population, the prevalence of chronic diseases, and the rise in outbreaks of infectious diseases have helped to expand the growth...
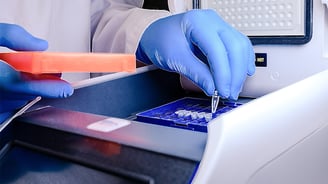
Membrane Materials and Applications for In Vitro Diagnostics
Industrially manufactured membranes play a vital role in the effectiveness of in vitro diagnostics instruments and procedures, such as protein assays and liquid/gas...

In Vitro Diagnostics Trends: Faster, Smarter, and More Personalized
According to a 2016 report in PLoS One, between 60 and 70% of medical decisions are made based on the results of in-vitro diagnostics (IVD) testing. Using the evaluation...
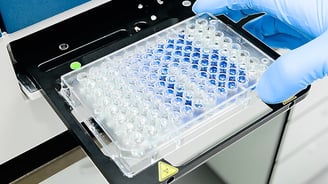
Common Material Challenges in Miniaturizing In Vitro Diagnostics
The miniaturization of diagnostic tests comes with several unique challenges. The aim is to transform the fast turnaround time and specificity of Lab on a Chip (LOC)...
%20-%20Tint%20100.png?width=106&name=Logo%20-%20Standard%20-%20Blue%20(270%20x%20149)%20-%20Tint%20100.png)